by Lisa E. Lynn
Biology Senior Seminar
Professor Trent Smith
Goshen College, Goshen, IN
November 26, 2001
For decades now, death and disease have driven the progress of technology. Through the advancements of science, many diseases have been made obsolete and many more are drawing closer and closer to being conquered. However, with all the diseases that we have defeated, more and more keep appearing. And old diseases that we thought we were protected against have made comebacks. An example of this is Foot and Mouth Disease. "Since 1930 the United States of America has prohibited the importation of livestock and fresh, chilled, or frozen meat from countries in which rinderpest or foot-and-mouth disease exist," (Publication 1343, 49). The United States is considered a Foot and Mouth Disease Free country. However, that does not mean that we have not been active in trying to get rid of FMD in other countries. There was an Argentine-United States Joint commission on FMD held in 1966. In a report on this meeting it was stated that, "The conditions under which the virus of foot-and-mouth disease (FMD) survives in animal tissues have long been matters of fundamental interest to all officials concerned with the prevention and control of the disease," (Publication 1343, 3). There was a CENTO Seminar on Viral Diseases held in Istanbul, Turkey on June 12-17, 1972. This seminar had a special emphasis on FMD and rinderpest-like diseases. A discussion of disease-free zones and the regulations for these zones was brought up (Girard 93). Some of the stated regulations included complete control of domestic livestock movement, traffic of persons to and from an area that has been quarantined should be restricted and if an outbreak would occur, no animals can be exported, all the animals in the infected area should be slaughtered and the area should remain in quarantine and clear of stock for at least one month, (Girard 93).
"FMD is caused by an aphthovirus of the family Picornavridae," (Aiello 457). This virus is spread both directly and indirectly. It is readily airborne and can be spread by the movement of animals, people and vehicles. "Infected animals have a large amount of aerosol virus in their exhaled air, which can infect other animals via the respiratory or oral routes," (Aiello 457). "All excretions and secretions from the infected animal contain virus, and virus may be present in milk and semen for up to 4 days before clinical signs appear," (Aiello 457). "Milk tankers carrying infected milk have been implicated in the spread of disease between farms, (Aiello 457).
FMD affects cloven-hoofed animals, which includes cattle, pigs, sheep, goats, buffalo, artriodactyl wildlife species, and all species of deer, antelope, elephant and giraffe (Aiello 457). The disease is characterized by fever, vesicles in the mouth and on the muzzle, teats and feet, and will result in death in young animals. Symptoms found in dairy cattle include a general malaise, lameness, secondary infections, declining milk yields, abortion, sterility, and death (Weir 1338). FMD does not pose an immediate, direct health risk for humans. "No human-to-human transmission has been recorded," (Brown 1463). The only recorded human case of FMD was in Great Britain in 1967 (Mayor 1085). A farm worker was, "…thought to have been infected by drinking contaminated milk," (Mayor 1085). His symptoms included a mild fever, sore throat, blisters on the palms of his hands, and weals on his tongue," (Mayor 1085). The man recovered within a few weeks and had no lasting health effects," (Mayor 1085).
Although FMD does not pose a direct health threat to the human population, it can cause a major economic disaster, especially for FMD free countries. There was a major outbreak of FMD in the United Kingdom just this past March. The tourist industry was losing millions every week and it was suggested that the general election be postponed (TB & Outbreaks 12). "For a country to be considered 'FMD-free' by international regulators, it must have an effective system of animal surveillance and disease reporting. The occurrence of even a single case of foot-and-mouth disease in a previously disease-free country results in an immediate ban on export trade," (Weir 1338). "An animal vaccine is available, but because current serologic tests cannot distinguish between infected and vaccinated animal, FMD-free countries will not import vaccinated animals, (Weir 1338). "The big problem with the vaccination is that some vaccinated animals only become weakly immune. This means they can still be infected and possibly pass the disease to other animals yet not show any obvious symptoms," (TB & Outbreaks 13). For this reason, lots of disease-free countries practice a policy of no vaccination so that they can continue to export. "In 1991 all European countries implemented a policy of nonvaccination in order to increase export opportunities," (Weir 1338). Because of the disastrous outbreaks both in Europe last year and in the United Kingdom this past summer, this decision for non-vaccination is coming under critical scrutiny.
There are several regulations imposed by the United States to halt the spread of an outbreak here if one should occur. They include the slaughter of all affected and in –contact susceptible animals, strict restrictions on movement of animals/vehicles around infected premises, the burning/burying of carcasses on or near premises, disinfection of buildings with mild acid or alkali and with fumigation, and then trace of the outbreak to determine its origins (Aiello 458-459). With regulations like these you would think that our country would be safely protected against the outbreak and spread of FMD and other livestock viruses. As a nation, we have safeguarded ourselves against FMD by enforcing strict export/import regulations. However, even the US is not impervious to outbreaks of strange and exotic diseases.
Since 1999, our country has been witness to the slow, but steady ambush of West Nile Virus, an exotic disease that has never before been seen in the Western hemisphere. "West Nile virus (WNV) was first isolated from the blood of a woman with a febrile disease in the West Nile district of Uganda in 1937," (Garmendia 223). "It was subsequently recovered from people, birds and mosquitoes in Egypt in the 1950s," (Garmendia 223). WNV is, "…recognized to be one of the most widespread of the flaviviruses, with a vast geographic distribution, which now includes North America," (Garmendia 223).
"West Nile virus first appeared in North America in the summer of 1999 in New York City causing 62 cases of human neurologic disease, 7 deaths, and leaving thousands of crows, other birds, and horses dead in its wake," (Craven 651). "WNV is a small single-stranded RNA virus of 40-nm size with a lipid envelope," (Garmendia 223). It is an arbovirus (arthropod-borne virus) and, “…a member of the Japanese encephalitis antigenic group within the family Flaviviridae,” (Garmendia 223). It has, "…been found in the Middle East and Europe along major bird migration flyways (aerial routs that migratory birds usually follow on their annual north/south migrations) that connect Europe, Africa, and the Middle East," (Craven 651). "WNV is transmitted between the natural bird reservoir hosts primarily by mosquitoes," (Craven 651). "In geographic areas such as Africa, the Middle East and Southwestern Asia, where the disease is endemic, the virus is maintained in nature in enzootic bird-mosquito-bird cycles, which are nearly impossible to eliminate," (Garmendia 223).
"Since West Nile virus can infect humans, birds, and mosquitoes, any of these hosts may have served as a vector for the introduction of the disease into the United States," (Tyler 1858). Several different propositions have been raised as to how WNV got into the United States in the first place. The notion that an infected human being came to America and spread the disease was quickly put to rest. "Humans are typically dead-end hosts for arboviruses, making it unlikely that an infected person traveling from an area where the virus is endemic was the initial source of infection," (Tyler 1858). Humans can be infected and may become ill, but they do not, "…develop a viremia that is sufficient for the continuation of a transmission cycle if bitten by another mosquito," (Craven 651). Another suggestion that perhaps West Nile was introduced by a virus-infected tick or mosquito, transported by ship or plane from an area where the infections is endemic is not impossible, but highly unlikely (Tyler 1858). The most likely way that West Nile was introduced into the United States was by an infected migratory or imported bird and that the infection was then spread to other animals and to humans by mosquitoes (Tyler 1858).
In the U.S., "…the initial outbreak in humans coincided with the deaths from West Nile virus of several thousand American crows and the deaths of exotic birds in the Bronx and Queens zoos," (Tyler 1858). Over the years there have been many outbreaks of West Nile around the globe. Outbreaks occurred in Israel (1951-1954, 1957), South Africa (1974), Romania and Morocco (1996), Tunisia (1997), and Italy (1998) with the most recent outbreaks occurring in Russia and Israel in 1999 and again in Israel and also in France in 2000 (Petersen, 611). West Nile has a widespread distribution in Africa, West Asia, and the Middle East. There have also been several sporadic cases of WNV in Belarus, Algiers, the Czech Republic, Azerbaijan, Central African Republic, the Congo, Egypt, Ethiopia, India, Madagascar, Nigeria, Pakistan, Senegal, Sudan and several European countries (Garmendia 223). The more recent outbreak in Algiers in 1994 resulted in fifty cases of WNV infection with a mortality rate of over ten percent (Garmendia 223). The outbreaks in southern Russia in the Astrakhan, Krasnodar and Volgograd regions resulted in the reports of around 1000 cases and 40 deaths (Garmendia 223).
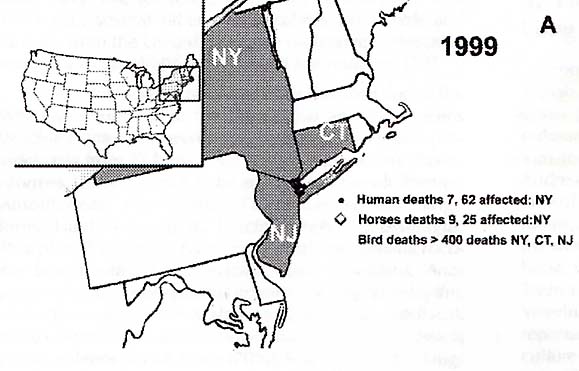
West Nile Virus was first detected in New York City, New York during the summer of 1999. "62 persons with WNV illness, including seven deaths, were detected in New York City and nearby New York counties," (MMWR, JAMA, 5/2/2001, 2188). (Refer to Figure 1) This is a twelve percent case fatality rate. "Veterinary pathologists at the Bronx Zoo and a wildlife pathologist in upstate New York suggested, independently, that the deaths of birds in southern New York might be related to the encephalitis of humans, both of which occurred during August and September," (Garmendia 225). "Hundreds of birds died in New York and Connecticut," (Garmendia 225). "From August to November 1999, 31 horses from Aquabogue, New York became ill and had neurologic signs," (Garmendia 226). "Finding the WNV in horses indicated that the disease had spilled over beyond wild birds to domestic species. Recognition that horses in the United States became infected with the WNV resulted in embargoes against horses from affected counties, especially for export to the European Union," (Garmendia 226).
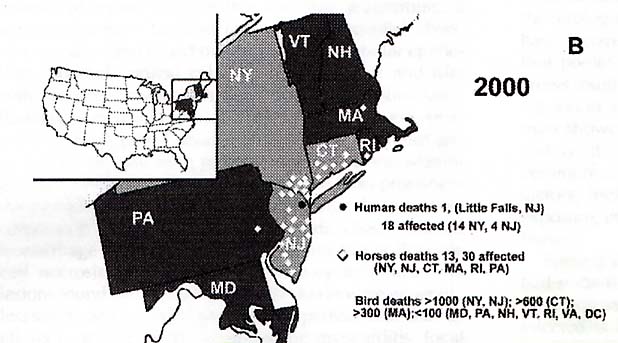
In 2000, "…21 persons were identified with acute WNV infection (14 in new York, six in New Jersey, and one in Connecticut), including two deaths (one each in New York and New Jersey)," (MMWR, JAMA, 5/2/2001, 2188). In 2000, the disease had spread from New York to Vermont, New Hampshire, Maryland, Rhode Island, Connecticut, New Jersey, Pennsylvania, and Maine (Figure 2) (Garmendia 225). "The reappearance of WNV in the spring and summer of 2000 confirmed earlier suspicions that the virus had survived the winter," (Garmendia 228). "An elderly man in Little Falls, New Jersey died of West Nile fever and at least 18 individuals had clinical illness in New York and New Jersey," (Garmendia 228). "As of October 2000 more than 20 horses with West Nile fever had died or been euthanized in New York, New Jersey, Connecticut, Pennsylvania, Massachusetts, and Rhode Island," (Garmendia 228). Also, more than 350 different pools of mosquitoes have tested positive to be carriers for WNV and it has been detected in bats, cats, raccoons, domestic rabbits, squirrels, and a chipmunk (Garmendia 228).
As of 2001, 62 cases of human neurologic disease, nine human deaths, hundreds of horse deaths, and thousands of bird deaths have been attributed to West Nile virus. Figure 3 and Figure 4 are maps that show the progress of the disease and areas of testing for the disease across the U.S. (http://cindi.usgs.gov/hazard/event/west_nile/west_nile.html). "During 2001, 25 human cases of WNV encephalitis have been reported in New York (6), Connecticut (5), Maryland (5), Florida (4), New Jersey (4), and Georgia (1); one death occurred in Georgia," (MMWR Weekly Update, 9/26-10/2/2001, 857). It also spread northward into Canada and outward to the Midwest portion of the United States.
With all of this widespread suffering and illness caused by West Nile Virus, you would think that people would be more alert to the possibility of infection. However, many of the symptoms of West Nile can be misconstrued as some other ailment. For all the cases that were reported, it is believed that there were likely to be 140 other infections that weren't reported due to the fact that people didn't realize what their ailments could potentially be (Henderson 22). The symptoms of West Nile virus in humans include gastrointestinal symptoms, muscle pains, headache, fever, flu-like symptoms, frank encephalitis and death. Symptoms of encephalitis include fever, confusion, disorientation, and/or coma, and any or all of these symptoms might be present in a person with West Nile fever (Craven 652). "There is no specific treatment for WNV infection or vaccine to prevent it. Treatment of sever illnesses includes hospitalization, use of intravenous fluid and nutrition, respiratory support, prevention of secondary infections and good nursing care," (CDC website). "Medical care should be sought as soon as possible for persons who have symptoms suggesting severe illness," (CDC website).
These same symptoms can also be found in animals. Some of the symptoms found in horses infected with WNV include, "…ataxia, weakness of limbs, difficulty in rising, muscle fasciculation, fever, facial paralysis, facial twitching, teeth grinding, and blindness," (Garmendia 226). Horses and birds are considered to be sentinel species for West Nile. If species of birds and horses are becoming ill in a specific area, then it is believed that it is a potential threat for humans in that area as well. In 1999, 25 confirmed cases of WNV were found on Long Island; 35 % were fatal (Ostlund 665). In 2000, 60 confirmed cases of West Nile Virus were reported; 38% of these cases were fatal (Ostlund 667). The most recent case of equine West Nile virus was reported this past week in Northern Indiana. It was found in a 7-year-old mare in the northcentral part of the state (The Farmer's Exchange 20). Laurent Couetil, an associate professor of veterinary clinical sciences at Purdue University said, "Horses should definitely be vaccinated before mosquito season next spring," (The Farmer's Exchange 20). As a result of this, vets in the surrounding area have been strongly cautioning horse owners to vaccinate their animals. Vaccinating horses now will help them to begin building antibodies and they should also receive a booster shot in the spring when mosquitoes start to reappear.
There is a vaccination for horses against West Nile virus. It is manufactured by Fort Dodge, a pharmaceutical company based in Fort Dodge, Iowa. This vaccine is a killed virus vaccine and aids the horse in making antibodies against the virus. It is questionable as to how well the vaccine works. So far it has been hard to tell whether it makes the animals completely immune or if it just helps them resist infection.
As of yet, there is no vaccine for humans, although steps are being taken to produce one. In a study conducted by scientists in the Department of Pathology and Laboratory Medicine at the University of Pennsylvania and scientists at the Viral Genomix in Philadelphia, a DNA vaccine was constructed and tested on mice. "In this study, a DNA vaccine encoding the WNV capsid protein was constructed, and the in vivo immune responses generated were investigated in DNA vaccine-immunized mice,"(Yang et al 809). To construct the DNA vaccine cassette, they cloned a full-length West Nile virus capsid protein sequence (Yang et al 813). They tested the resulting plasmid structure by sequence analysis to verify what they had created. Then they tested it using Western blot analysis to see if the plasmids expressed the West Nile virus capsid protein. This testing showed that the plasmids did in fact express the sought after protein, so they then immunized mice by injecting the vaccine intramuscularly. They collected serum samples to see if the mice were producing antibodies for West Nile. "The mice immunized with pDCNA3 control did not show any Cp-specific antibody response; however, we observed potent Cp-specific antibody responses from mice immunized with pCWNVCp," (Yang et al 813). Although it has yet to be made into an official DNA vaccine, these preliminary findings could lead us to a vaccine for humans. "DNA vaccination is an appropriate tactic for vaccine development to prevent WNV infection. DNA immunization affords several potential advantages over traditional vaccination strategies, " (Yang et al 816).
Besides just creating a vaccine for humans, other steps have been taken to fight the spread of West Nile. An abundance of spraying was conducted throughout the suburbs and boroughs of New York City. Spraying was made possible in Quebec Canada after the virus broke out there. The spraying was made possible by Bill 15, which passed through the Quebec National Assembly in June of 2001 (Sibbald 463). The bill, "…overrides other legislation, including municipal pesticide bans (there are 10 in the Montreal area alone) and Ministry of Environment rules requiring special permit for aerial spraying," (Sibbald 463). This overhaul of legislation has the environmentalists of the Quebec area up in arms. They argue that the risks associated with spraying Malathion, the pesticide that Quebec was planning to use, do not justify its use. "Malathion is approved for use in both the US and Canada and is used routinely to control mosquitoes in Winnipeg. It is highly toxic to fish and aquatic invertebrates. Last summer it killed thousands of lobsters on the US eastern seaboard, spawning lawsuits from commercial fishermen," (Sibbald 463). With these kind of potential outcomes, it is questionable whether or not spraying will be considered as a long-term solution.
There are several surveillance systems in place to keep an eye on the spread of the virus. The CDC has an entire website devoted to following the spread of the disease and there is another organization called ArboNET, that is an enhanced human and animal surveillance system which was designed specifically to monitor the geographic spread of West Nile virus in the United States and to identify areas at increased risk for human infections of WNV (MMWR JAMA, 9/22-29/2001, 910). Also, quite a bit of funding has been put towards halting WNV. "In New York, Governor George Pataki's administration is spending more than $20 million on surveillance, control, and research this year, while the U.S. Centers for Disease Control and Prevention (CDC) is giving the state another $3.9 million to deal with the outbreak," (Enserink 1289).
"The current epidemic of WN virus in New York City is unprecedented
and underscore the ease with which pathogens can move among the population
centers of the world," (Lanciotti 2337). "It is not yet known how
the virus was introduced, nor how long it has been in the United States.
The extent of its geographic distribution remains a mystery, as does the
long-term impact it may have on human and animal health," (Lanciotti 2337).
In an editorial note issued by the CDC it was stated that, "The detection
of WNV in Florida and southern Georgia in 2001, extends substantially the
known distribution of this virus," (MMWR, JAMA, 9/22-29/2001, 910).
Authorities hoped that the virus would be killed off as temperatures dropped
and mosquitoes died off. However, when it reappeared just as strong
in 2000, that hope was abandoned. Now that it is reached the Southern
parts of the US, it is feared that it will become established there due
to the year round warm weather. Also, there is a potential that infected
mosquito eggs may hatch out next spring and migratory birds might spread
the disease even further.
No matter how technologically advanced we may become, we must always
remember that we are vulnerable to infection and disease. "The emergence
(or introduction) in human population of West Nile virus, Ebola virus,
Lyme disease and particularly HIV all illustrate how susceptible humans
still are to infectious diseases," (Dobson 1011). Death and disease
will always be a driving force behind progress. Even with ongoing
surveillance and preventative measures, we cannot predict the outcome of
this outbreak of West Nile in America. We do not know what kind of
long-term affects it will have on the wildlife within our nation or on
the human population. At this point, all we can do is wait and see.

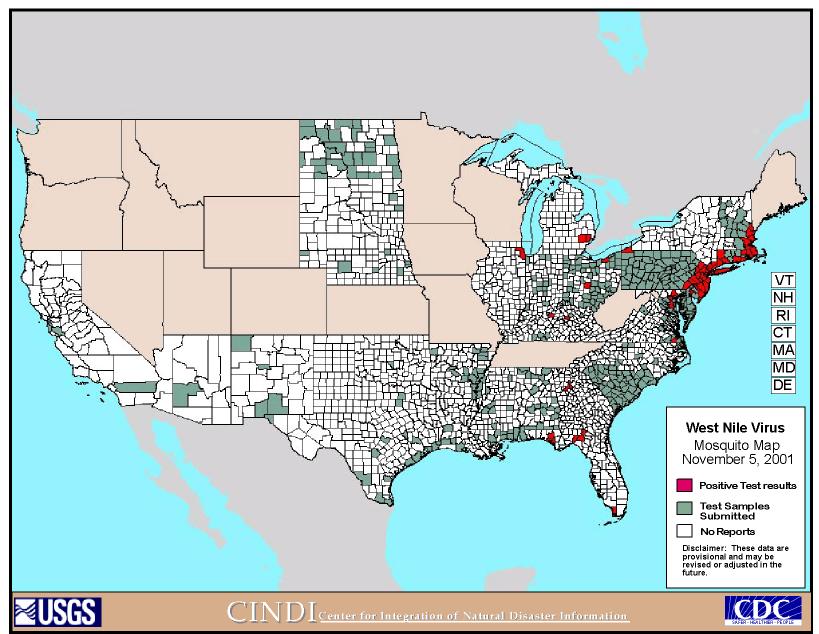
(http://cindi.usgs.gov/hazard/event/west_nile/west_nile.html)
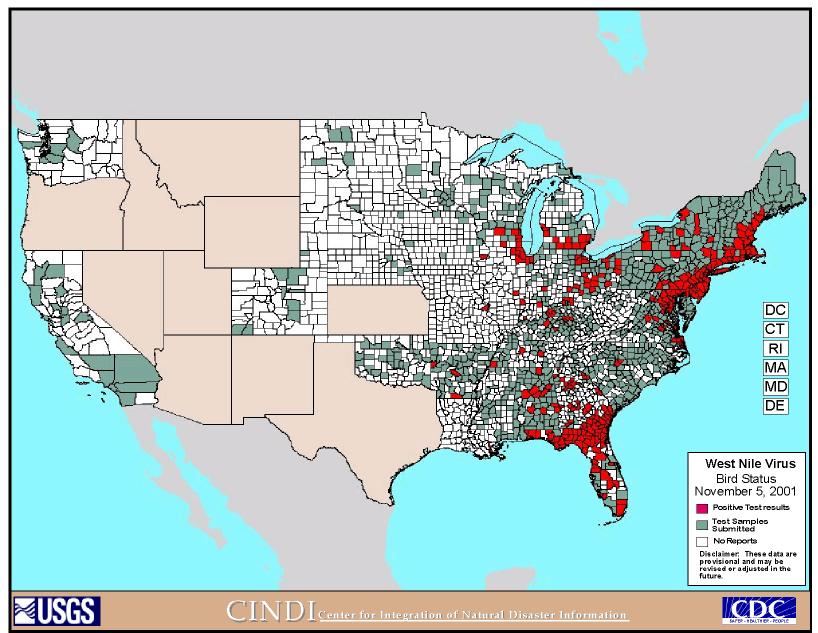
(http://cindi.usgs.gov/hazard/event/west_nile/west_nile.html)
"Human West Nile Virus Surveillance – Connecticut, New Jersey, and New York, 2000." (MMWR) JAMA: Journal of the American Medical Association. Vol. 285, No. 17. p. 2188-2190.
"Weekly Update: West Nile Virus Activity—United States, September 26-October 2, 2001." MMWR: Morbidity & Mortality Weekly Report. Vol. 50, Issue 39. 10/5/2001. p. 857-866.
“A Report of the Argentine-United States Joint Commission on Foot-and-Mouth Disease.” Publication 1343, National Academy of Sciences. National Research Council, Washington, DC. 1966
“Foot-and-Mouth Disease: Countries Debate Vaccination Policy.” TB & Outbreaks Week. 5/8/01
“West Nile Virus Activity – Eastern United States, 2001.” MMWR: JAMA. (CDC Statement) Vol. 286, No. 8. August 22/29, 2001.
"West Nile Virus Found in Northern Indiana Horse." The Farmer's Exchange. November 9, 2001.
Aiello, Susan E. Editor. The Merck Veterinary Manual, 8th Edition. Whitehouse Station, NJ, 1998.
Brown, David W G. “Foot and Mouth Disease in Human Beings.” Lancet. Vol. 357, Issue 9267. 5/12/01
CENTO Seminar on the Control & Eradication of Viral Diseases in the CENTO Region. Central Treaty Organization. Istanbul, Turkey, 1972.
Craven, Robert B. M.D. & John T. Roehrig PhD. “West Nile Virus.” JAMA. Vol. 286, No. 6. August 8, 2001
Dobson, A. et al. “Emerging Infectious Pathogens of Wildlife.” Philosophical Transactions of the Royal Society London Biological Sciences. Edited by MEJ. Woolhouse and C. Dye. October 2001.
Enserink, Martin. "West Nile Researchers Get Ready for Round Three." Science. Volume 292, No. 5520. May 18, 2001.
Enserink, Martin. “Groups Race to Sequence and Identify New York Virus.” Science. Vol. 286, Oct. 8, 1997.
Garmendia, Antonio E., et al. “The West Nile Virus: Its Recent
Emergence in North America.” Microbes and Infection. March
2001.
Henderson, C.W. "West Nile Virus: Analysis Suggests Infection
Outbreak in U.S. Was Greater than Previously Thought." TB & Outbreaks
Week. 8/21/2001-8/28/2001, p 22.
http://cindi.usgs.gov/hazard/event/west_nile/west_nile.html
Lanciotti, R.S. et. al. "Origin of the West Nile Virus Responsible for an Outbreak of Encephalitis in the Northeastern United States." Science. Vol. 286, No. 5448. 12/17/1999. p. 2333-2337.
Mayor, Susan. “UK Investigates Possible Human Cases of Foot and Mouth Disease.” BMJ. Vol. 322, Issue 7294. 5/5/01
Ostlund, Eileen N., et al. “Equine West Nile Encephalitis, US.” Emerging Infectious Diseases. Vol. 7, Issue 4. Jul/Aug 2001.
Petersen, Lyle R. & John T. Roehrig, Guest Editors. “West Nile Virus: A Reemerging Global Pathogen.” Emerging Infectious Diseases. Vol. 7, Issue 4. Jul/Aug 2001.
Sibbald, Barbara. "Quebec Clear Way for Use of Aerial Pesticides to Combat West Nile Virus." CMAJ: Canadian Medical Association Journal. Vol. 165, Issue 4. 8/21/2001, p. 463.
Tyler, Kenneth L., M.D. “West Nile Virus Encephalitis in America.” The New England Journal of Medicine. Vol. 344, No. 24. June 14, 2001.
Weir, Erica. “Foot and Mouth Disease in Animals and Humans.” Canadian Medical Association Journal. Vol. 164, Issue 9. 5/1/01
Yang, Joo-Sung, et al. “Induction of Potent Th1-Type Immune Responses
from a Novel DNA Vaccine for West Nile Virus New York Isolate (WNV-NY1999).”
The Journal of Infectious Diseases. Vol. 184, 2001.